Abstract
Introduction. In follicular lymphoma (FL), no prognostic index has been built based on a cohort of patients treated only with initial immunochemotherapy. Given the emergence of new biomolecular and metabolic prognostic factors in FL, an easy-to-compute and reliable scoring system utilizing such variables could aid in trial stratification and routine clinical evaluation.
Methods. The primary endpoint for model building was progression-free survival (PFS). For discovery, we used the large prospective PRIMA trial cohort of 1,135 patients treated with initial R-chemotherapy +/- R-maintenance. Variables considered for model building included age, sex, performance status, B symptoms, stage, number of nodal and extranodal sites involved, LDH, hemoglobin, longest diameter of the largest lymph node, presence of effusion and compression syndrome, circulating lymphoma cells, platelet count, serum albumin, bone marrow involvement, and ß2m. For validation, we combined 175 patients with high tumor burden FL from the R-CHVP+interferon arm of treatment from the FL2000 trial and 304 patients prospectively enrolled in the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) who were treated upfront with immunochemotherapy. Event-free survival (EFS) was the endpoint available in the validation cohort.
For model building, we used a two-step approach based on resample Cox regression and conditional inference trees analyses to identify 3 patient groups of approximately equal size and experiencing differential outcome in terms of PFS. For comparison with the FLIPI, model's discrimination was computed using theConcordance Probability Estimates (CPE) based upon Harrell's c-index. All statistical tests were 2-sided. A P value <.05 was considered statistically significant.
Results. The final simplified score based on the discovery set was called the PRIMA-PI (PRIMA-prognostic index) and comprised 3 risk categories: high (ß2m > 3 mg/L), low (ß2m ≤ 3 mg/L without bone marrow involvement) and intermediate (≤ 3 mg/L with bone marrow involvement).
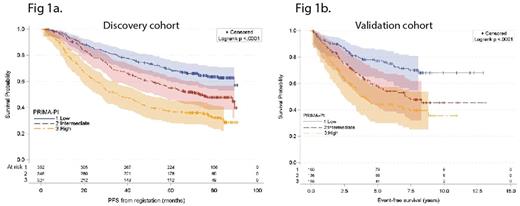
In the discovery cohort, the PRIMA-PI was highly discriminatory with 5-year PFS of 69% (95%CI: 64-73%), 55% (49-60%) and 37% (32-42%) in the low-, intermediate- and high-risk groups respectively (P <0.0001, Figure 1a). The PRIMA-PI outperformed the FLIPI by concordance probability (CPE 0.577 for the FLIPI and 0.604 for the PRIMA-PI). The PRIMA-PI was superior to the FLIPI both in the observation and the maintenance arm. The PRIMA-PI and the FLIPI similarly predicted OS with a 5-yr OS of 93% in the low/intermediate-risk category versus 84% in the high-risk category for the FLIPI and 93% and 81% for the PRIMA-PI respectively (P <0.0001 for each). However, the PRIMA-PI displayed better predictive performance than the FLIPI for cause-specific death. The PFS at 24 months (PFS24) was a strong post-treatment prognostic parameter for subsequent OS in the discovery cohort and the PRIMA-PI was a better predictor of PFS24 than the FLIPI. For patients without disease progression before 24 months, 5-year OS from the 24-month landmark or the risk-defining event was 92% (95% CI, 80-94%), compared to 63% (95% CI, 57-70%) for patients who progressed in the first 24 months (P <0.0001).
In the validation cohort, the PRIMA-PI remained highly discriminatory: 5-year EFS was 77% (69-83%), 57% (48-66%) and 44% (35-52%) in the low-, intermediate- and high-risk groups respectively (P <0.0001, Figure 1b). Finally, the PRIMA-PI was at least as discriminant as the FLIPI in terms of statistical performances in the validation cohort (CPE 0.605 for the FLIPI and 0.606 for the PRIMA-PI).
Conclusion. We developed and validated a new prognostic tool comprising only 2 parameters (bone marrow involvement and ß2m) which are easily measured clinically. The PRIMA-PI is a new and easy-to-compute prognostic index for patients treated upfront with immunochemotherapy. This could serve as a basis for building more sophisticated and integrated biomolecular scores.
Bachy: Amgen: Honoraria; Sandoz: Consultancy, Honoraria; Mundipharma: Research Funding; Roche/Genentech: Consultancy, Honoraria, Research Funding. Estell: Janssen: Membership on an entity's Board of Directors or advisory committees. Delmer: Abbvie: Consultancy, Honoraria; Janssen: Honoraria; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Belada: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants , Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants , Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants , Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau. Ansell: Celldex: Research Funding; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Merck: Research Funding; Affimed: Research Funding. Lamy: Roche: Consultancy, Honoraria. Cerhan: Janssen: Other: Scientific Advisory Board (REMICADELYM4001); Janssen: Other: Multiple Myeloma Registry Steering . Salles: Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.